| 货号: 11951 |
| 产品全名: 人 S100A9 蛋白 |
| 规格: 10/50/100 µg |
| 基因符号 MIF;NIF;P14;CAGB;CFAG;CGLB;L1AG;LIAG;MRP14;60B8AG;MAC387;S100-A9 |
| 目标蛋白: S100A9 |
| UNIPROT ID: P06702 |
| 描述: Recombinant Human S100A9 Protein with N-terminal human Fc tag |
| 背景: The protein encoded by this gene is a member of the S100 family of proteins containing 2 EF-hand calcium-binding motifs. S100 proteins are localized in the cytoplasm and/or nucleus of a wide range of cells, and involved in the regulation of a number of cellular processes such as cell cycle progression and differentiation. S100 genes include at least 13 members which are located as a cluster on chromosome 1q21. This protein may function in the inhibition of casein kinase and altered expression of this protein is associated with the disease cystic fibrosis. This antimicrobial protein exhibits antifungal and antibacterial activity. [provided by RefSeq, Nov 2014] |
| 物种/宿主: HEK293 |
| 分子量: The protein has a predicted molecular mass of 39.4 kDa after removal of the signal peptide. The apparent molecular mass of hFc-S100A9 is approximately 35-55 kDa due to glycosylation. |
| 分子特征: hFc(Glu99-Ala330) S100A9(Met1-Pro114) |
| 纯化: The purity of the protein is greater than 95% as determined by SDS-PAGE and Coomassie blue staining. |
| Formulation & Reconstitution: Lyophilized from nanodisc solubilization buffer (20 mM Tris-HCl, 150 mM NaCl, pH 8.0). Normally 5% – 8% trehalose is added as protectants before lyophilization. |
| 储存和运输: Store at -20°C to -80°C for 12 months in lyophilized form. After reconstitution, if not intended for use within a month, aliquot and store at -80°C (Avoid repeated freezing and thawing). Lyophilized proteins are shipped at ambient temperature. |
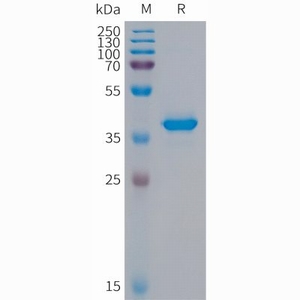
Figure 1.Human S100A9 Protein, hFc Tag on SDS-PAGE under reducing condition. |




